Antiscalant dosing vs. softening system
Advantages for process water treatment in outpatient clinical settings
Proper sterilization of surgical instrumentation represents one of the most relevant processes in clinical practice. Depending on the type of operations and their number, proper reprocessing requires a high degree of process reliability for the reprocessing steps involved. In addition to sterile reprocessing, the protection of the instruments plays an elementary role in this process.
The water treatment required for this purpose in accordance with the state of the art and knowledge (taking into account the guidelines of the DGSV and AKI) includes a wide variety of process steps that must ensure this.
The starting material for the sterilization process is drinking water, the composition of which varies greatly from site to site, particularly with regard to water hardness and dissolved constituents.
During the operation of membrane systems (reverse osmosis systems), particles can be deposited on the membrane surface (scaling). In this process, the pores of the membrane are blocked and the effective filter area is reduced. As a result, the water treatment plant requires more energy and the quality of the permeate deteriorates with increasing operating time. Elaborate cleaning procedures are required to free the membrane elements from the blockages or replacement of the membrane is necessary. To ensure the protection of the reverse osmosis, a preparation of the water is needed. For this purpose, softening systems or antiscalant dosing systems are often used.
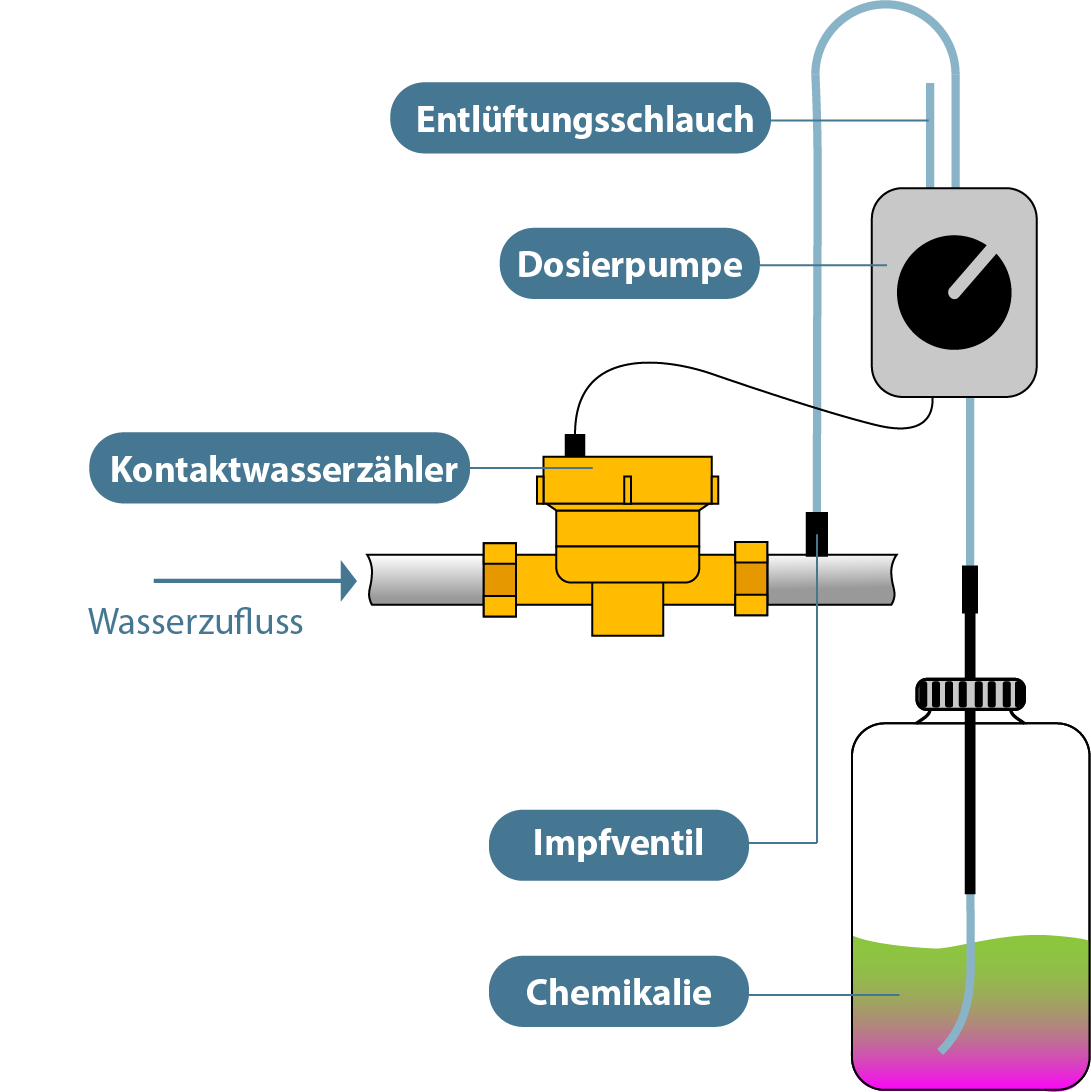
The antiscalant dosages have the disadvantage for the clinical area in contrast to the softening plants that chemicals are added to the water, which complex the metal cations (e.g. calcium and magnesium) and thus prevent the blocking of the membrane. The chemicals may only be handled by skilled personnel, who are not on site in most outpatient clinics. As a result, work safety cannot be guaranteed at all times. Furthermore, a hazard assessment must be prepared for the chemicals of Antiscalant Dosage to account for the proper storage of the liquid.
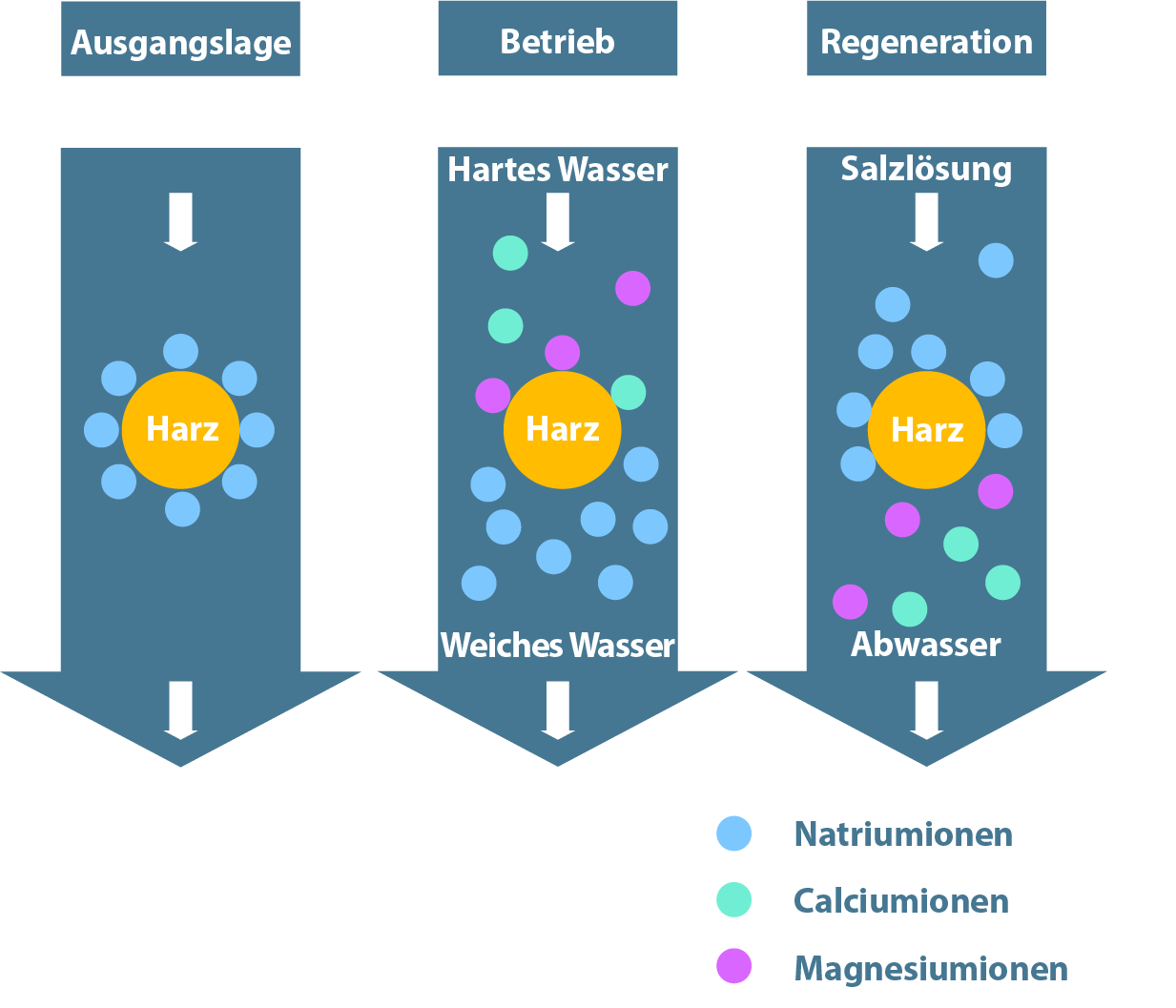
In contrast, the process of softening plant does not require the use of agents labeled as hazardous substances, as it is a natural principle. For the coworkers no danger proceeds in this connection in handling the softener salt. Also the storage does not represent a special hurdle. The softener removes the magnesium and calcium in the water on the basis of ion exchange by an appropriate amount of sodium ions. The water thus treated subsequently contains more sodium ions and no calcium and magnesium ions. The concentration of all other ions remains unchanged. This process can produce soft water without chemicals, which prevents blocking.
From safety aspects and the advantages in handling for the caring staff, the use of a softening plant is recommended.